Rare Neurological Disease Treatment Market Expands Across USA, Europe, APAC & Saudi Arabia to 2035
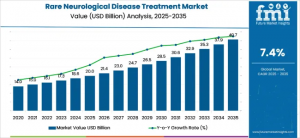
The global rare neurological disease treatment market will grow from USD 20.0B in 2025 to USD 40.7B by 2035, driven by gene therapy and precision medicine.
GERMANY, November 7, 2025 /EINPresswire.com/ -- The global rare neurological disease treatment market is set for strong and sustained growth, bolstered by advancements in precision medicine, gene therapy, and expanded clinical research collaborations. Valued at USD 20.0 billion in 2025, the market is projected to reach USD 40.7 billion by 2035, growing at a CAGR of 7.4% over the forecast period. The sector generated USD 18.75 billion in revenue in 2024, demonstrating accelerating investment and scientific momentum.
Rare neurological disorders, while individually uncommon, collectively affect a substantial segment of the global population. Studies indicate that 3.5% to 5.9% of the global population—approximately 260 to 450 million people—live with a rare disease, and nearly half of these conditions are neurological in nature. Growing awareness, earlier diagnosis, and advanced genomic tools are expanding the pool of patients eligible for therapeutic intervention.
Explore trends before investing — request a sample report today!:- https://www.futuremarketinsights.com/reports/sample/rep-gb-6445
Key Market Growth Drivers
Advances in targeted therapies are reshaping treatment landscapes. Technologies such as CRISPR gene-editing, RNA-based therapies, monoclonal antibodies, and refined biologic delivery systems are enabling disease-modifying approaches rather than symptomatic care. Swift regulatory approvals through orphan drug programs in the USA, Europe, and selected APAC markets also encourage accelerated commercialization.
Moreover, public-private partnerships and patient advocacy groups are prompting broader trial enrollment, improved access, and increased funding. These efforts support drug development for conditions historically underserved due to small patient populations and high research costs.
Regional Expansion Across Major Markets
• United States remains dominant with strong R&D capital expenditure, a robust biotech presence, and structured reimbursement systems. The market here is expected to continue steady growth at 7.4% CAGR.
• Europe, led by Germany, the UK, and France, is benefiting from national rare disease frameworks and investments in genomic research, contributing to 9.0% growth in Germany alone.
• Asia-Pacific shows the fastest momentum, particularly China (9.5% CAGR) and Japan (9.3% CAGR), fueled by innovation hubs, growing diagnostic capacity, and government funding.
• Saudi Arabia and GCC markets are investing in neurological centers of excellence and rare disease registries, strengthening access to specialty therapies and supporting clinical trials in the Middle East.
Market Segmentation Insights
• Drug Class: Anti-depressants remain the leading segment, accounting for 26% share in 2025, supported by psychiatric comorbidity in conditions such as ALS, Huntington’s disease, and multiple sclerosis.
• Route of Administration: Oral formulations are expected to hold 72% market share in 2025, driven by patient convenience and lower treatment delivery costs.
• Indication Focus: Amyotrophic Lateral Sclerosis (ALS) will represent 30% of the market in 2025, as aging populations and enhanced diagnostic awareness expand patient identification.
• Distribution Channel: Hospital pharmacies will lead, growing at 7.7% CAGR, due to the specialized handling requirements of gene therapies and biologics.
Click Here to Purchase the Report:- https://www.futuremarketinsights.com/checkout/6445
Industry Developments
• August 2024 – The NHS approved Belzutifan for Von Hippel-Lindau disease, marking a milestone in targeted tumor management.
• December 2024 – Novartis announced a licensing partnership of up to USD 2.9 billion for Huntington’s disease therapy development.
• January 2025 – Johnson & Johnson acquired Intra-Cellular Therapies for USD 14.6 billion, strengthening its neurological treatment pipeline.
Leading Companies in the Market
Major players include Pfizer Inc., Novartis Pharmaceuticals, Merck & Co., Johnson & Johnson Services, Bayer AG, GlaxoSmithKline, Sanofi S.A., AbbVie Inc., Teva Pharmaceuticals, and F. Hoffmann-La Roche Ltd. Tier-1 manufacturers collectively account for 52.2% of global market share.
Latest Therapy Area Reports:-
Medical Eye Shield Film Market
https://www.futuremarketinsights.com/reports/medical-eye-shield-film-market
Medical Far Infrared Therapy Device Market
https://www.futuremarketinsights.com/reports/medical-far-infrared-therapy-device-market
Kids Splint Market
https://www.futuremarketinsights.com/reports/kids-splint-market
Why Choose FMI Empowering Decisions that Drive Real-World Outcomes:- https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Sudip Saha
Future Market Insights Inc.
+1 347-918-3531
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.